Monica Vetter
Professor and Chair of Neurobiology and Adjunct Professor of Ophthalmology and Visual Sciences
Chromatin Modifiers in Development, Microglia in Retinal Development and Disease

Molecular Biology Program
Education
B.S. McGill University, Canada
Ph.D. University of California, San Francisco
Research
My laboratory is focused on understanding the molecular pathways controlling neural development and degeneration in the vertebrate neural retina. The retina is one of the most accessible parts of the central nervous system, and is of critical importance since disorders of eye development can lead to congenital blindness, while degeneration of retinal neurons can cause progressive loss of vision at later ages.
Chromatin Modifiers in Retinal Development
In the developing retina we are studying how eye tissues are patterned and how retinal progenitors are directed to adopt specific retina cell fates. Recently we have been focusing on how gene expression and fate decisions during eye development are modulated by chromatin remodeling factors via histone modifications. We have shown that the histone modifier Ezh2 is required to maintain transcriptional integrity of retinal progenitors, and preserve their capacity for proliferation. Ezh2 is also required to coordinate the timing of differentiation and generate the appropriate complement of late retinal cell types. We are now investigating additional epigenetic regulators to obtain a more complete understanding of how chromatin modification regulates gene expression during development. Ultimately, we seek to reveal general principles governing the development of neural stem cells and progenitors, which may inform efforts to harness these cells for the treatment of disease and injury of the nervous system.
Microglia in Retinal Development and Neurodegeneration
Microglia are the resident immune cells of the central nervous system and are abundant throughout the neural retina. They infiltrate the neural retina near the onset of neurogenesis, but their role in retinal development is poorly understood. We are investigating the properties of microglia in the developing retina, and assessing how they influence progenitor proliferation, differentiation, and neuronal survival.
Microglia also play complex roles during neurodegeneration. To investigate this, we are probing the mechanisms underlying glaucoma, a neurodegenerative disease of the retina that is characterized by progressive loss of retinal ganglion cells leading to blindness. Using an established mouse model for glaucoma, we have used live imaging and ex vivo analysis and found recruitment and activation of microglia at very early stages of the disease. Microglia are the resident immune surveillance cells of the CNS, and are exquisitely sensitive to neuronal stress and injury. We are testing the role of microglia in neuronal decline, and defining the signals leading to their recruitment and activation with disease progression. We are using gene therapy vectors and mouse knockouts to target key pathways and assess the impact on disease progression. Our ultimate goal is to identify molecular pathways that can be targeted to slow or prevent blindness in glaucoma.
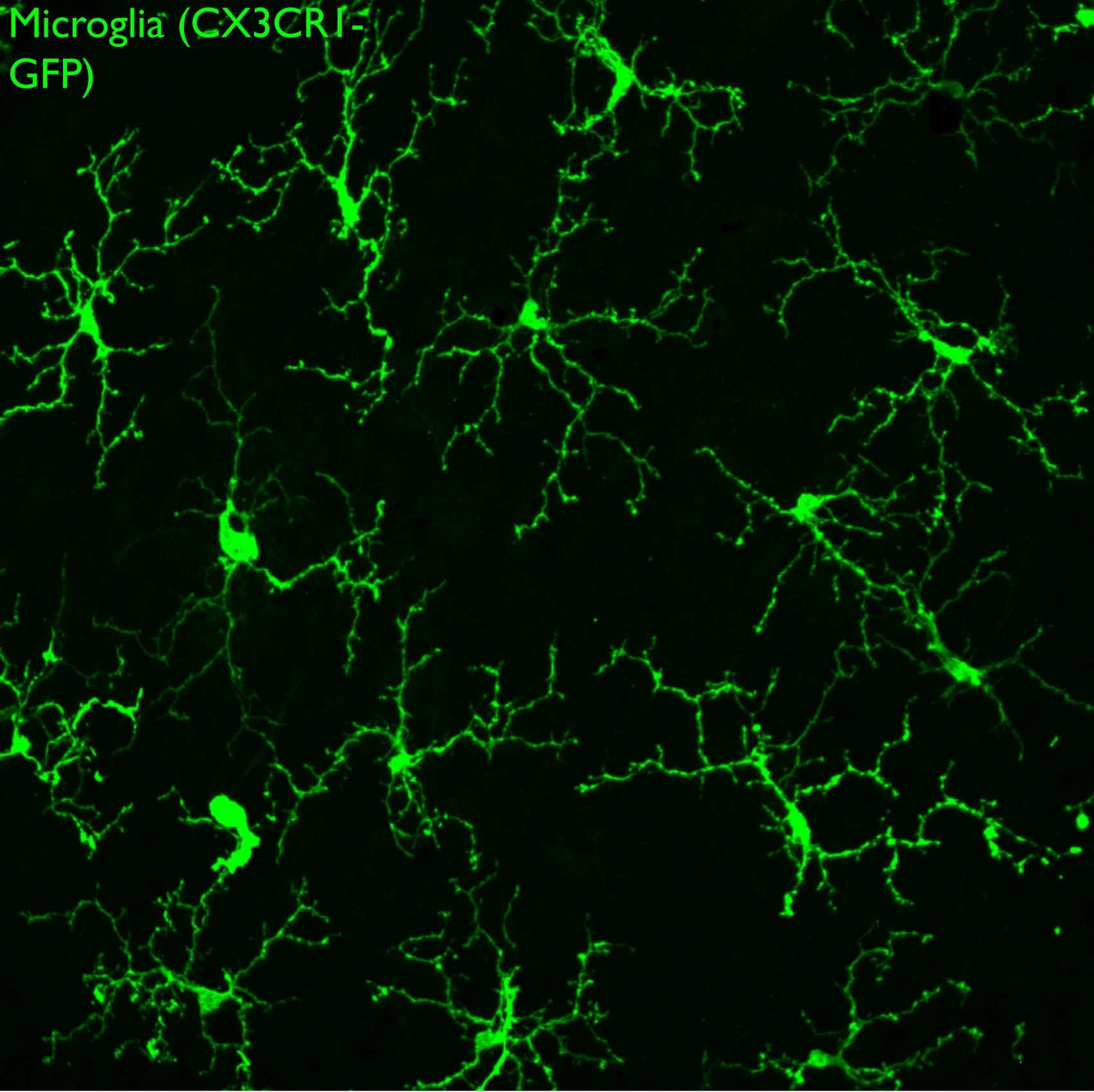
GFP-labeled microglia tile the surface of the retina. Confocal image of a retina whole mount preparation.
References
- Breen, K.T., Anderson, S.R., Steele, M.R., Calkins, D.J., Bosco, A., Vetter, M.L. (2016) Loss of fractalkine signaling exacerbates axon transport dysfunction in a chronic model of glaucoma. Front. Neurosci., Nov 24;10:526. PMCID: PMC5123443.
- Zhang, J. Taylor, R.J., LaTorre, A., Wilken, M.S., Cox, K.E., Reh, T.A., Vetter, M.L. Ezh2 maintains retinal progenitor proliferation, transcriptional integrity, and the timing of late differentiation. Dev. Biol, 403(2):128-38. PMCID: PMC4469612.
- Bosco, A., Romero, C.O., Breen, K.T., Chagovetz, A.A., Steele, M.R., Ambati, B.K., Vetter, M.L. (2015) Neurodegeneration severity is anticipated by early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis Model Mech, 8(5):443-455. PMCID: PMC4415894.
- Willardsen M, Hutcheson DA, Moore KB, Vetter ML (2014) The ETS transcription factor Etv1 mediates FGF signaling to initiate proneural gene expression during Xenopus laevis retinal development. Mechanisms of Development 131:57-67.
- Aldiri I, Moore KB, Hutcheson DA, Zhang J, Vetter ML (2013) Polycomb repressive complex PRC2 regulates Xenopus retina development downstream of Wnt/β-catenin signaling. Development 140(14):2867-2878.
- Aldiri I, Vetter ML (2012) PRC2 during vertebrate organogenesis: a complex in transition. Developmental Biology 367(2):91-99.
- Willardsen M, Hutcheson DA, Moore KB, Vetter ML (2014) The ETS transcription factor Etv1 mediates FGF signaling to initiate proneural gene expression during Xenopus laevis retinal development. Mechanisms of Development 131:57-67.
- Aldiri I, Moore KB, Hutcheson DA, Zhang J, Vetter ML (2013) Polycomb repressive complex PRC2 regulates Xenopus retina development downstream of Wnt/β-catenin signaling. Development 140(14):2867-2878.
- Bosco A, Crish SD, Steele MR, Romero CO, Inman DM, Horner PJ, Calkins DJ, Vetter ML (2012) Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS One 7(8):e43602
- Aldiri I, Vetter ML (2012) PRC2 during vertebrate organogenesis: a complex in transition. Developmental Biology 367(2):91-99.
- Green YS, Vetter ML (2011) EBF factors drive expression of multiple classes of target genes governing neuronal development. Neural Development 6:19
- Bosco A, Steele MR, Inman DM, Horner PJ, Vetter ML (2011) Early microglia activation in a muse model of chronic glaucoma. Journal of Comparative Neurology 519(4):599-620.
- Aldiri I, Vetter ML (2009) Characterization of the expression pattern of the PRC2 core subunit Suz12 during embryonic development of Xenopus laevis. Developmental Dynamics 238(12):3185-3192.
- Agathocleous M, Iordanova I, Willardsen MI, Xue Y, Harris WA, Vetter ML, Moore KB (2009) A directional Wnt/β-catenin-Sox2-Proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136(19):3289-3299.
- Willardsen MI, Suli A, Marsh-Armstrong N, Chien C-B, Brown NL, Moore KB, Vetter ML (2009) Temporal regulation of Ath5 gene expression during Xenopus eye development. Developmental Biology 326(2):471-481.
- Soto I, Oglesby E, Buckingham BP, Son JL, Roberson ED, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N (2008) Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. Journal of Neuroscience 28(2):548-561.
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML (2008) Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Investigative Ophthalmology & Visual Science 49(4):1437-1446.
- Hutcheson DA, Hanson MI, Moore KB, Le TT, Brown NB, Vetter ML (2005) bHLH-dependent and -independent modes of Ath5 gene regulation during retinal development. Development 132(4):829-839.
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML (2005) Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 46(1):23-36.
