Hongchao Guo
Assistant Professor of Cardiothoracic Surgery and Adjunct Assistant Professor of Human Genetics
Stem Cell Differentiation, Cardiovascular Disease, Genetics, Epigenetics, Heart Development & Regeneration
Molecular Biology Program
Biological Chemistry Program
Education
Ph.D. Nankai Univer
Postdoctoral - Stanford Cardiovascular Institute
Research
Dr. Guo's laboratory is dedicated to unraveling the genetic and epigenetic mechanisms that underlie cardiovascular development and diseases, employing stem cell models. The overarching research objective of the lab is to pioneer precision medicine approaches for patients with cardiovascular disease (CVD) by integrating molecular/cellular biology with cutting-edge next-generation sequencing and genome editing technologies. Specifically, the focus lies on two main areas: heart development and regeneration, and cardiovascular disease modeling.
In the realm of heart development and regeneration, the research delves into the intricate processes involved in embryonic heart formation, which culminate in the establishment of the four-chambered heart. Deviations from this developmental trajectory can lead to cardiac developmental disorders, impacting approximately 40,000 births annually in the United States alone. Human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) offer invaluable models for studying cardiac development due to their human origin and ability to generate human cardiac tissue. However, the precise roles and mechanisms of genetic and epigenetic regulation governing cardiac development and lineage specification/growth remain elusive. Leveraging advancements in next-generation sequencing and stem cell technologies, the lab has identified novel factors such as long non-coding RNA (e.g., BANCR) and epigenetic drivers (e.g., SETD7) that play pivotal roles in heart development. Additionally, novel reporter tools (e.g., TBX5Clover2 and NKX2-5TagRFP double reporter) have been developed to facilitate the study of cardiac development. Moreover, despite the limited regenerative capacity of the adult human heart, understanding and enhancing its regenerative potential hold promise for treating cardiovascular diseases. Insights gained from human 2D and 3D stem cell models of heart development will be translated into strategies for heart regeneration, particularly in the context of cardiovascular disease and heart failure.

(BANCR promotes cardiomyocyte migration during heart development)
In the arena of cardiovascular disease modeling, the focus is on addressing the complexity and heterogeneity of cardiovascular diseases (CVDs). The conventional approach is to treat all cardiac diseases, regardless of etiologies, as one disease, by lowering cholesterol, inhibiting beta-adrenergic and calcium channel pathways, and managing blood pressure. However, not all patients respond to these treatments; this can be attributed to CVD's heterogeneous nature and a lack of understanding of the genetic and environmental differences contributing to CVD susceptibility. Precision medicine for CVD patients with genetic variants holds promise as a new approach toward more effective prevention and treatment by understanding physiological mechanisms and causal relevance. Directed differentiation of human iPSCs into cardiovascular cells, such as cardiomyocytes and endothelial cells, has enabled the generation of patient-specific cardiovascular cells. Using iPSC-derived cardiovascular cells, the lab has elucidated mechanisms underlying genetic (e.g., ALDH2*2) and environmental (e.g., alcohol) factors' adverse effects on cardiovascular health. The lab aims to further explore how genetic variations contribute to susceptibility to environmental factor-related CVDs using iPSC models, with the ultimate goal of identifying precise treatment strategies.
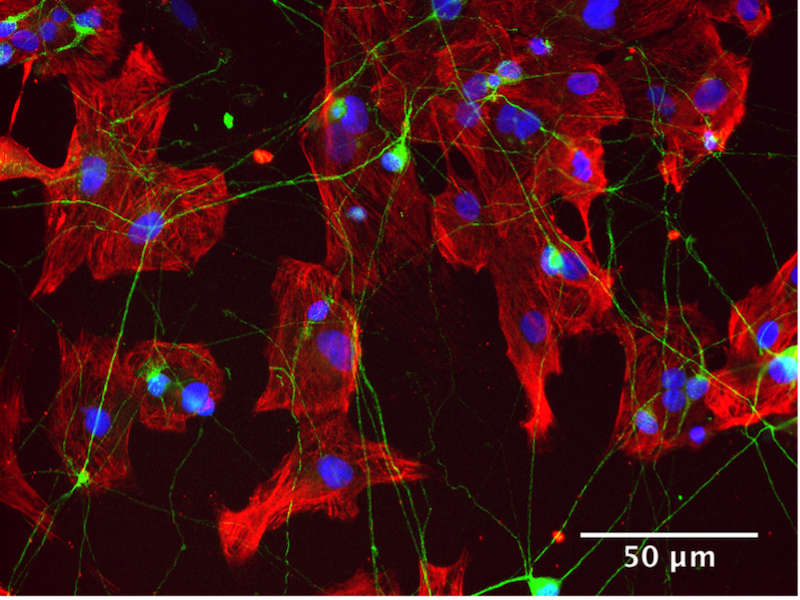
iPSC-derived neurons (green) and cardiomyocyte (red)
References (Selected Publications)
- Guo HC*, Yu X, Liu Y, Paik DT, David T Paik, Justesen JM, Chandy MJ, Jahng JW, Zhang TJ, Wu WJ, Rwere F, Pokhrel S, Simon DJ, Manhas A, Zhang A, Chen CH, Rivas MA, Gross ER, Mochly-Rosen D, Wu JC. SGLT2i ameliorates endothelial dysfunction associated with the common ALDH2 alcohol flushing variant. Science Translational Medicine. 2023 Jan 25;15(680):eabp9952. doi: 10.1126/scitranslmed.abp9952. PMID: 36696485.
- Yang BX*, El Farran CA*, Guo HC * (co-first), Yu T*, Fang HT, Wang HF, Schlesinger S, Seah YF, Goh GY, Neo SP, Li Y, Lorincz MC, Tergaonkar V, Lim TM, Chen L, Gunaratne J, Collins JJ, Goff SP, Daley GQ, Bard FA, Loh YH. Systematic identification of factors for provirus silencing in embryonic stem cells. Cell. 2015; 163(1):230-45. PMID: 26365490; PMCID: 4686136.
- Guo HC*, Liu LC, Nishiga M, Wu JC. Deciphering pathogenicity of variants of uncertain significance with CRISPR-edited iPSCs. Trends in Genetics. 2021; 37, 1109-1123. PMID: 34509299; PMCID: PMC8578372.
- Wilson KD*, Ameen M*, Guo HC* (co-first), Abilez OJ, Tian L, Mumbach MR, Diecke S, Qin X, Liu Y, Yang H, Ma N, Gaddam S, Cunningham NJ, Gu M, Neofytou E, Prado M, Hildebrandt TB, Karakikes I, Chang HY, Wu JC. Endogenous retrovirus-derived lncRNA BANCR promotes cardiomyocyte migration in humans and non-human primates. Dev Cell. 2020 July 28: S1534-5807(20)30580-3. PMID: 32763147; PMCID: PMC7529962.
- Guo HC*, Tian L*, Zhang JZ, Kitani T, Paik DT, Lee WH, Wu JC. Single-cell RNA-sequencing of human embryonic stem cell differentiation delineates effects of nicotine on embryonic development. Stem Cell Reports. 2019; 12(4):772-786.

