Gabe Nagy
Assistant Professor of Chemistry
Bioanalytical Separations, Mass Spectrometry, Carbohydrates

Biological Chemistry Program
Education
B.S. Creighton University
Ph.D. Indiana University
Research
Recently, human milk oligosaccharides (HMOs) have been implicated for their roles in promoting the healthy development of the brain-gut microbiota axis of a neonate. However, their numerous potential structural permutations make their accurate analyses and characterization increasingly difficult. To better understand how, why, and what, specific HMOs influence the relationship between the brain, gut, and immune system of an infant, better analytical tools are desperately needed. To solve this puzzle, we will focus on the development of a bioanalytical workbench consisting of a new multidimensional separations platform to be used in conjunction with various solution and gas-phase chemical probes to enable better separation and characterization of this highly diverse class of unconjugated glycans. Additionally, the use of accelerated reactions in microdroplets will permit rapid syntheses of biomedically-relevant glycan standards. This proposed bioanalytical toolbox will pave the way for de novo human milk oligosaccharide characterization, enabling advances in disease, clinical, and microbiome research.
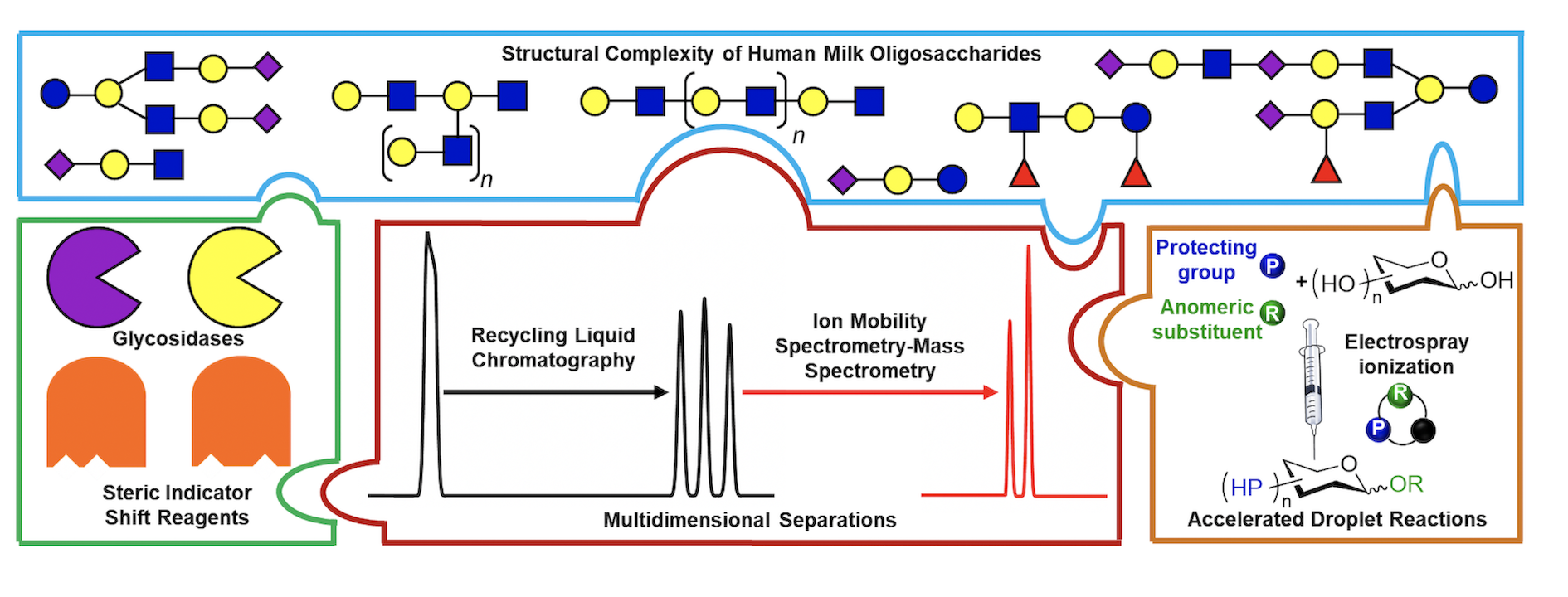
Core research topics include:
- Implementation and application of a new recycling liquid chromatography-ion mobility spectrometry-mass spectrometry (recycling LC-IMS-MS) platform for high-resolution human milk oligosaccharide analyses
- Design of a chemical probes toolbox to enable more confident identification of unknown glycans
- Development of accelerated reactions in microdroplets for rapid syntheses of carbohydrate building blocks and biomedically-relevant glycans
- Cyclic ion mobility separations of isotopologues and isotopomers
References
-
Peterson, T. L.; Nagy, G. “Toward Sequencing the Human Milk Glycome: High-Resolution Cyclic Ion Mobility Separations of Core Human Milk Oligosaccharide Building Blocks.” Analytical Chemistry, 2021, DOI: 10.1021/acs.analchem.1c00942.
-
Nagy, G.; Kedia, K.; Attah, I. K.; Garimella, S. V. B.; Ibrahim, Y. M.; Petyuk, V. A.; Smith, R. D. “Separation of b-Amyloid Tryptic Peptide Species with Isomerized and Racemized L-Aspartic Residues with Ion Mobility in Structures for Lossless Ion Manipulations.” Analytical Chemistry, 2019, 91, 4374–4380.
-
*Garimella, S. V. B.; *Nagy, G.; Ibrahim, Y. I.; Smith, R. D. “Opening New Paths for Biological Applications of Ion Mobility-Mass Spectrometry using Structures for Lossless Ion Manipulations.” Trends in Analytical Chemistry, 2019, 116, 300–307. (*Co-first author).
-
Nagy, G.; Attah, I. K.; Garimella, S. V. B.; Tang, K.; Ibrahim, Y. M.; Baker, E. S.; Smith, R. D. “Unraveling the Isomeric Heterogeneity of Glycans: Ion Mobility Separations in Structures for Lossless Ion Manipulations.” Chemical Communications, 2018, 54, 11701–11704.
-
Gaunitz, S.; Nagy, G.; Pohl, N. L. B.; Novotny, M. V. “Recent Advances in the Analysis of Complex Glycoproteins.” Analytical Chemistry, 2017, 89, 389–413.
-
*Nagy, G.; *Peng, T.; Kabotso, D. E. K.; Novotny, M. V.; Pohl, N. L. B. “Protocol for the
-
Purification of Protected Carbohydrates: Toward Coupling Automated Synthesis to Alternate-Pump Recycling High-Performance Liquid Chromatography.” Chemical Communications, 2016, 52, 13253–13256. (* Co-first author).
-
Nagy, G.; Pohl, N. L. B. “Complete Hexose Identification with Mass Spectrometry.” Journal of the American Society for Mass Spectrometry. 2015, 26, 677–685.
