Shannon Odelberg
Research Associate Professor of Internal Medicine and Adjunct Associate Professor of Neurobiology
Cellular Plasticity, Regeneration

Molecular Biology Program
Education
B.S. Weber State University
Ph.D. Virginia Commonwealth University
Research
My research has primarily focused on two major topics: 1) the role of the small GTPase ADP ribosylation factor 6 (ARF6) in regulating signaling pathways that are involved in cancer, vascular permeability, and inflammation and 2) elucidating the cellular and molecular basis of regeneration in newts. Both of these avenues of research have provided us with insights into the molecular, cellular, and extracellular mechanisms that control cell plasticity, proliferation, and migration under pathological conditions.
The Role of ARF6 in Regulating Signaling Pathways that Drive Cancer and Inflammation
The activation of the small GTPase ARF6 has been implicated in promoting several pathological processes related to tumor establishment, growth, and metastasis, vascular leak, and inflammation [1]. We have shown that ARF6 controls the trafficking of key oncogenic proteins to the appropriate intracellular locations where they enhance signaling. For example, in uveal melanoma, activation of ARF6 promotes the trafficking of oncogenic GNAQ from the plasma membrane to cytoplasmic vesicles where PLC/PKC, Rho/Rac, YAP, and b-catenin signaling are enhanced [2]. Inhibition of ARF6 reverses the trafficking process, decreases signaling, and reduces tumor establishment and growth in an orthotopic xenograft mouse model of uveal melanoma, suggesting that ARF6 might be a good therapeutic target for this deadly cancer. We have also shown that inhibiting ARF6 in cutaneous melanoma reduces metastasis to the lungs in a xenograft mouse model of cutaneous melanoma [3] and that ARF6 activates PIK3 in melanoma to induce prometastatic state [4]. We continue to study these tumors, while expanding our efforts to also include the role of ARF6 in other cancers, such as glioblastoma and malignant sarcomas.
ARF6 also plays a role in vascular stability and inflammation. The activation of ARF6 promotes vascular instability and fluid leak into surrounding tissues during inflammatory processes, while the inhibition of ARF6 stabilizes the vasculature and reduces harmful vascular leak. Using cell culture assays and preclinical animal models, we have shown that inhibiting ARF6 activation in diseases and disorders associated with vascular leak and chronic and acute inflammation, such as diabetic retinopathy, rheumatoid arthritis, acute lung injury (a model for acute respiratory distress syndrome), and sepsis, reduces pathology and enhances survival rates in the lethal conditions [1,5]. We have shown that, similar to its role in cancer, activation of ARF6 promotes the trafficking of key proteins to appropriate intracellular locations where signaling is enhanced. We continue to explore the molecular mechanisms by which ARF6 exerts its effects on such a diverse array of inflammatory processes.
Elucidating the Cellular and Molecular Basis of Regeneration in Newts
For many years, my laboratory focused on the cellular and molecular basis of regeneration in newts. A newt has the remarkable ability to regenerate lost or injured structures and organs, including its limbs, tail, spinal cord, heart ventricle, retinas, lenses, optic nerves, upper and lower jaws, intestines, and parts of the brain. Many of the progenitor cells required for regeneration are created de novo by the dedifferentiation of mature cells located near the site of injury. This degree of cellular plasticity is unique to organisms with marked regenerative abilities and is not observed in mammals. Over the years, my laboratory and our collaborators have identified several factors involved in inducing cellular plasticity and have shown that a transitional extracellular matrix can induce cell behaviors that are required for normal regeneration, including cell dedifferentiation, proliferation, and migration[6-9].
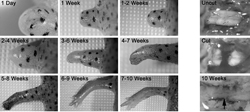
Newt limb and spinal cord regeneration. Left panel. An adult newt forelimb regenerates in 7-10 weeks following amputation through the stylopod. Right panel. An adult newt can regenerate a completely transected spinal cord and regain function in less than 10 weeks. Arrow indicates lesion site (shown as a gap in the middle photograph) that contains regenerated neural tissue at 10 weeks post-injury.
References
-
Zhu W, Shi DS, Winter JM, Rich BE, Tong Z, Sorensen LK, Zhao H, Huang Y, Tai Z, Mleynek TM, Yoo JH, Dunn C, Ling J, Bergquist JA, Richards JR, Jiang A, Lesniewski LA, Hartnett ME, Ward DM, Mueller AL, Ostanin K, Thomas KR, Odelberg SJ, Li DY. Small GTPase ARF6 controls VEGFR2 trafficking and signaling in diabetic retinopathy. J Clin Invest. 2017 Dec 1;127(12):4569-4582. doi: 10.1172/JCI91770. Epub 2017 Oct 23. PMCID: PMC5707163
-
Grossmann, A.H., Zhao, H., Jenkins, N., Zhu, W., Richards, J.R., Yoo, J.H., Winter, J.M., Rich, B., Mleynek, T.M., Li, D.Y., and Odelberg, S.J. (2016). The small GTPase ARF6 regulates protein trafficking to control cellular function during development and in disease. Small GTPases, 1-12.
-
Yoo, J.H., Shi, D.S., Grossmann, A.H., Sorensen, L.K., Tong, Z., Mleynek, T.M., Rogers, A., Zhu, W., Richards, J.R., Winter, J.M., Zhu, J., Dunn, C., Bajji, A., Shenderovich, M., Mueller, A.L., Woodman, S.E., Harbour, J.W., Thomas, K.R., Odelberg, S.J., Ostanin, K., and Li, D.Y. (2016). ARF6 Is an Actionable Node that Orchestrates Oncogenic GNAQ Signaling in Uveal Melanoma. Cancer Cell 29, 889-904.
-
Grossmann, A.H., Yoo, J.H., Clancy, J., Sorensen, L.K., Sedgwick, A., Tong, Z., Ostanin, K., Rogers, A., Grossmann, K.F., Tripp, S.R., Thomas, K.R., D'Souza-Schorey, C., Odelberg, S.J., and Li, D.Y. (2013). The small GTPase ARF6 stimulates beta-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci Signal 6, ra14.
-
McGann, C.J., Odelberg, S.J., and Keating, M.T. (2001). Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci U S A 98, 13699-13704.
-
Odelberg, S.J., Kollhoff, A., and Keating, M.T. (2000). Dedifferentiation of mammalian myotubes induced by msx1. Cell 103, 1099-1109.
-
Calve, S., Odelberg, S.J., and Simon, H.G. (2010). A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol 344, 259-271.
-
Vinarsky, V., Atkinson, D.L., Stevenson, T.J., Keating, M.T., and Odelberg, S.J. (2005). Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol 279, 86-98.
