Eric W. Schmidt
Distinguished Professor of Medicinal Chemistry, Adjunct Professor of Biological Sciences, and Adjunct Professor of Chemistry
Natural products from animals and the animal microbiome; biosynthesis; synthetic biology; antibiotics; neuroactive compounds; metagenomics

Molecular Biology Program
Biological Chemistry Program
Education
B.S. University of California, San Diego
Ph.D. University of California, San Diego
Research
Chemistry plays a large role in how animal biology, including in communication, defense, physiology, symbiosis, and many other areas. Our lab seeks to understand how chemistry is used, how compounds are made, and how they might be applied to biotechnology and pharmaceuticals. Our projects cover diverse forms of life, with a major focus on marine invertebrates such as tunicates, mollusks, and other creatures that live in the sea.
Research Area 1: Biosynthesis in Animals and the Animal Microbiome
Reading a textbook, you might think that the chemicals found in animals are well understood, but we have yet to know the structures of most compounds. We don’t know who makes the compounds – the animal, its microbial symbionts, or others – or what enzymes and genes might be involved in their biosynthesis. Our lab seeks to uncover the structures of important compounds from animals, and to determine how they are made. To do so, we use interdisciplinary tools including metagenomics, bioinformatics, organic spectroscopy, enzymology, and others. The aim is to uncover the hidden chemical niches of life and to understand the connections between organisms in nature and their specialized chemicals.
Research Area 2: Synthetic Biology Approaches to the Chemistry of Life
In animals, chemicals are put to use to tackle a wide variety of challenges. Perhaps because of these diverse challenges, the chemistry found in animals is diverse and varied. By following the distribution and evolution of chemicals found in animals, potential rules for their engineering can be discerned. Our goal is to find those rules in nature, to test them experimentally, and to use them in the rational construction of recombinant small molecules. There are many reasons to do so. For example, many potential therapeutic compounds from the ocean are limited because they are found only rarely on coral reefs or other delicate ecosystems. By bringing the genes into the lab and manipulating them, we can produce those compounds and make analogs without the expense and environmental damage of harvesting coral reefs. It becomes possible to create new chemicals using only your imagination and the enzymes in hand.
We have focused so far mostly on ribosomally synthesized natural products (RiPPs) and polyketides, but many other types of compounds are under study currently.
Research Area 3: Drug Design and Discovery
The rich and diverse chemicals found in animals are bioactive and compatible with animal metabolism. In short, they are potential drugs. We apply this rich resource to discover new natural compounds, with a current focus on neuronal diseases such as pain and infectious diseases. We also apply our synthetic biological approach to the discovery and optimization of new drug lead compounds.
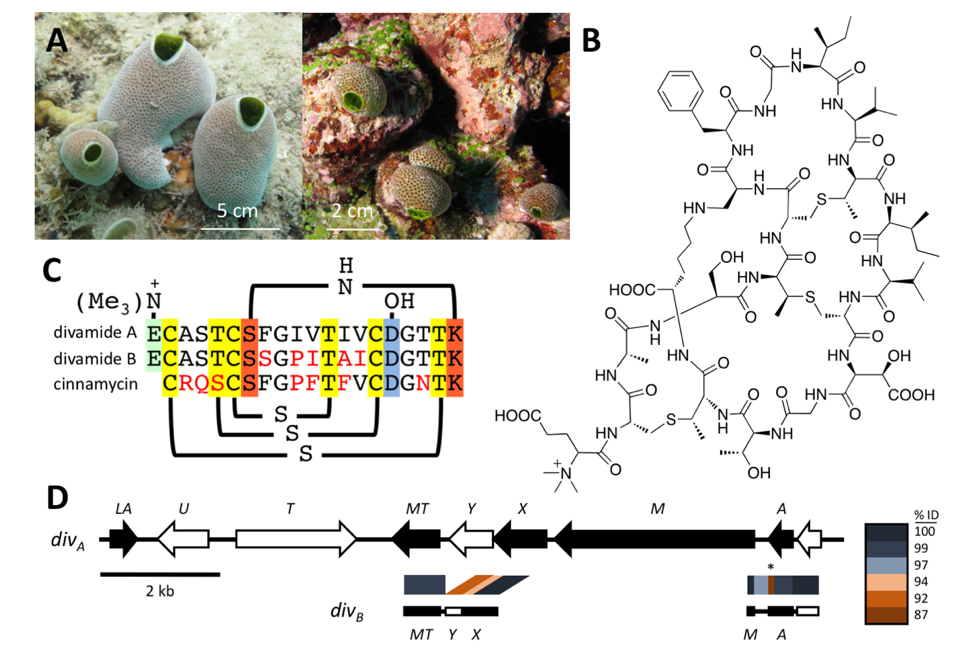
Figure. (Smith et al., Nature Chemical Biology 2018). A metagenomic approach to discovering new pharmaceuticals. A Marine animals (tunicates) were obtained in the Eastern Fields of Papua New Guinea. They contained potent anti-HIV compounds, but we could only find those in vanishingly small quantities. B Using a combination of metagenome sequencing, synthetic biology, and spectroscopy, we defined the structures of divamides, the active anti-HIV compounds from the tunicates. C The active compounds were RiPP peptides with different sequences that affected the activity against HIV. D The biosynthetic gene cluster from divamides was discovered using metagenomic sequencing. The pathway was expressed in E. coli bacteria, enabling us to supply the compound for pharmacological study.
References (Selected Publications)
- Li, F.; Lin, Z.; Torres, J.P.; Hill, E.; Li, D.; Townsend, C.A.; Schmidt, E.W. Sea urchin polyketide synthase SpPks1 produces the naphthalene precursor to echinoderm pigments. J. Am. Chem. Soc. 2022, epub.
- Scesa, P. D.; Lin, Z.; Schmidt, E.W. Ancient defensive terpene biosynthetic gene clusters in the soft corals. Nat. Chem. Biol. 2022, epub.
- Gu, W.; Zheng, Y.; Pogorelov, T.; Nair, S. Schmidt, E.W. Control of nucleophile chemoselectivity in cyanobactin YcaO heterocyclases PatD and TruD. ACS Chem. Biol. 2022, epub.
- Paguigan, N.D.; Yan, Y.; Karthikeyan, M.; Chase, K.; Leavitt, L.; Carter, J.; Lim, A.; Memon, T.; Christensen, S.; Bentzen, H.B.; Schmitt, N.; Reilly, C.; Teichert, R.W.; Raghuraman, S.; Olivera, B.M.; Schmidt, E.W. The tunicate metabolite 2-(3,5-diiodo-4-methoxyphenyl)ethan-1-amine targets voltage-gated ion channels of vertebrate sensory neurons. ACS Chem. Biol. 2021, 16, 1654-1662.
- Paguigan, N.D.; Leavitt, L.; Tun, J.O.; Lin, Z.; Dowell, C.; Deering-Rice, C.E.; Lim, A.L.; Karthikeyan, M.; Reilly, C.A.; Raghuraman, S.; Mcintosh, J.M.; Olivera, B.M.; Schmidt, E.W. Nicotinic acetylcholine receptor partial antagonist polyamides from tunicates and their predatory sea slugs. ACS Chem. Neuro. 2021, 12, 2693-2704.
- Miller, B.W.; Lim, A.; Lin, Z.; Bailey, Aoyagi, K.L.; Fisher, M.A.; J.; Barrows, L.R.; Manoil, C.; Schmidt, E.W. ;* Haygood, M.G.* Shipworm symbiosis ecology-guided discovery of an antibiotic that kills colistin-resistant Acinetobacter. Cell Chem. Biol.2021. *co-corresponding
- Torres, J. P.; Lin, Z.;* Watkins, M.; Florez, P. F.; Baskin, R. P.; Elhabian, S.; Safavi-Hemami, H.; Taylor, D.; Tun, J.; Saguil, N.; Fang, Y.; Concepcion, G. P.; Yanagihara, A. A.; McArthur, J. R.; Tae, H.-S.; Finol-Urdaneta, R. K.; Özpolat, B. D.; Olivera, B.M.; Schmidt, E. W.* Small molecule mimicry hunting strategy in the imperial cone snail, Conus imperialis. Sci. Adv.2021, 7, eabf2704. *co-corresponding
- Sarkar, S.; Gu, W.; Schmidt, E. W. Expanding the chemical space of synthetic cyclic peptides using a promiscuous macrocyclase from prenylagaramide biosynthesis. ACS Catal. 2020, 10, 7146-7153, PMC7805243
- Torres, J. P.; Lin, Z.; Winter, J.M.; Krug, P. J.; Schmidt, E. W. Animal biosynthesis of complex polyketides in a photosynthetic partnership. Nat. Commun. 2020, 11, 2882. PMC7280274.
- Torres, J.P.; Schmidt, E.W. The biosynthetic diversity of the animal world. J. Biol. Chem. 2019 epub.
- Lin, Z.; Kakule, T.B.; Reilly, C.A.; Beyhan, S.; Schmidt, E.W. Secondary metabolites of Onygenales fungi exemplified by Aioliomyces pyridodomos. J. Nat. Prod.2019 epub.
- Gu, W.; Sardar, D.; Pierce, E.; Schmidt, E.W. The role of multiple cassettes in cyanobactin RiPP biosynthesis. J. Am. Chem. Soc.2018, 140, 16213-16221.
- Morita, M.; Hao, Y.; Jokela, J.K.; Sardar, D.; Lin, Z.; Sivonen, K.; Nair, S.K.*; Schmidt, E.W.* Post-translational tyrosine geranylation in cyanobactin biosynthesis. J. Am. Chem. Soc. 2018, 140, 6044–6048. *co-corresponding
- Smith, T.E.; Pond, C.D.; Pierce, E.; Harmer, Z.P.; Kwan, J.; Zachariah, M.; Harper, M.K.; Wyche, T.P.; Matainaho, T.K.; Bugni, T.S.; Barrows, L.R.; Ireland, C.M.; Schmidt, E.W. Accessing chemical diversity from the uncultivated symbionts of small marine animals. Nat. Chem. Biol.2018, 14, 179-185.
