Paul Sigala
Associate Professor of Biochemistry
Faculty Co-advisor, University of Utah SACNAS
Malaria Parasite Biochemistry and Cell Biology, Heme and Iron Metabolism, Organelle Metabolism, Regulation, and Adaptation

Molecular Biology Program
Biological Chemistry Program
Education
B.S. University of California San Diego
Ph.D. Stanford University
Postdoc Washington University in St. Louis
Research
Malaria is an ancient scourge of humanity and one of the deadliest infectious diseases worldwide. All clinical symptoms of malaria arise during parasite infection of erythrocytes, and this developmental stage can be readily cultured in vitro to enable in-depth study of the molecular factors and cellular features that equip Plasmodium parasites to survive and proliferate within host erythrocytes.
Our research has two general goals:
- To develop and apply diverse cellular, genetic, and biochemical tools to uncover general metabolic principles and adaptations governing the unique biology of P. falciparum parasites during infection of human red blood cells.
- To apply this knowledge to develop novel strategies to target this virulent pathogen.
Our work is highly interdisciplinary and spans multiple areas of cell biology, genetics, chemical biology, protein biochemistry, and biophysics. For many proteins, we use Crispr/cas9-based genome editing to tag endogenous parasite proteins for localization and trafficking studies and for conditional regulation of protein expression in blood-stage parasites. We exploit the power of in vitro biochemistry to interrogate the structural and functional properties of purified proteins and reconstituted biochemical pathways. As appropriate, we also carry out parallel studies in bacteria and yeast or mammalian cells to develop tools and to compare and contrast general metabolic principles in discrete prokaryotic and eukaryotic organisms.
In the Sigala lab, we value broadly different backgrounds and perspectives, inclusive teamwork, collaboration, mentorship, and combining intellectual forces to tackle pressing and fascinating biomedical problems at the frontier of our knowledge and understanding of Plasmodium and broader eukaryotic biology. I am passionate about leveraging my cross-disciplinary training to mentor students to be rigorous, creative, and curiosity-driven scientists.
Organelle Function and Adaptation
Plasmodium parasites contain a single mitochondrion and a curious chloroplast-like organelle, called the apicoplast. These organelles carry out key metabolic processes but have unusual structural and functional features relative to other eukaryotic organisms. We are applying a suite of genetic, chemical, and biophysical probes to dissect and understand the functional properties and metabolic adaptations of discrete biochemical pathways within these organelles across multiple stages of sexual and asexual parasite development. Ultimately, our goals are to broaden fundamental knowledge of fascinating and divergent parasite biology and to uncover novel therapeutic opportunities.
Heme Metabolism
We study the cellular mechanisms by which parasites acquire, traffic, and utilize the essential cofactor heme.
- Parasites express a complete heme biosynthesis pathway but do not require its activity during blood-stage infection, suggesting an ability to scavenge host heme. We are developing and applying genetically encoded heme biosensors and chemical probes to dissect the pathways of heme trafficking and acquisition within parasites.
- Plasmodium parasites require heme as a cofactor for cytochrome-dependent electron transport in the mitochondrion. We employ a battery of in-parasite and in vitro approaches to dissect and understand the maturation of the critical cytochrome c and c1 components. To clarify the broader landscape of heme utilization by parasites, we are taking cellular and biochemical approaches to test the functions of diverse non-canonical heme proteins identified within the highly divergent parasite genome.
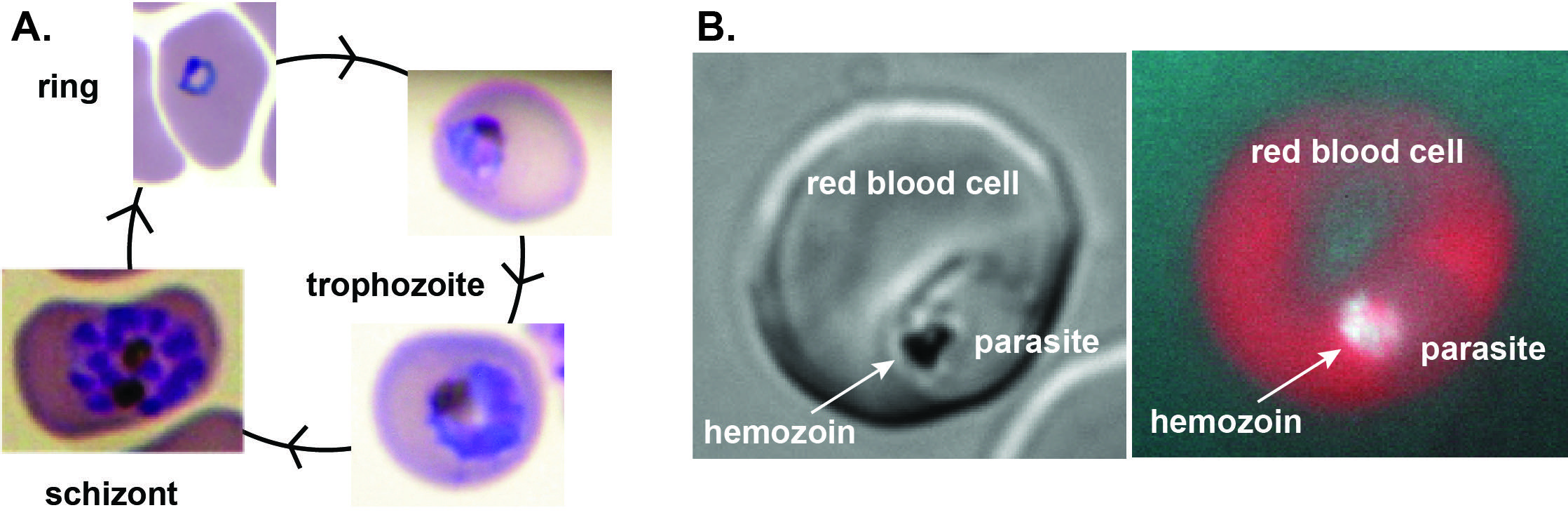
A) Asexual parasites grow and multiply with a 48-hour life cycle within red blood cells,
where they degrade ~80% of hemoglobin and liberate free heme crystallized as hemozoin.
B) Left: Bright field image of trophozoite within host red blood cell. Right: Red fluorescence
after stimulating porphyrin biosynthesis within the infected red blood cell, with
autofluorescence from hemozoin crystals in the parasite digestive vacuole.
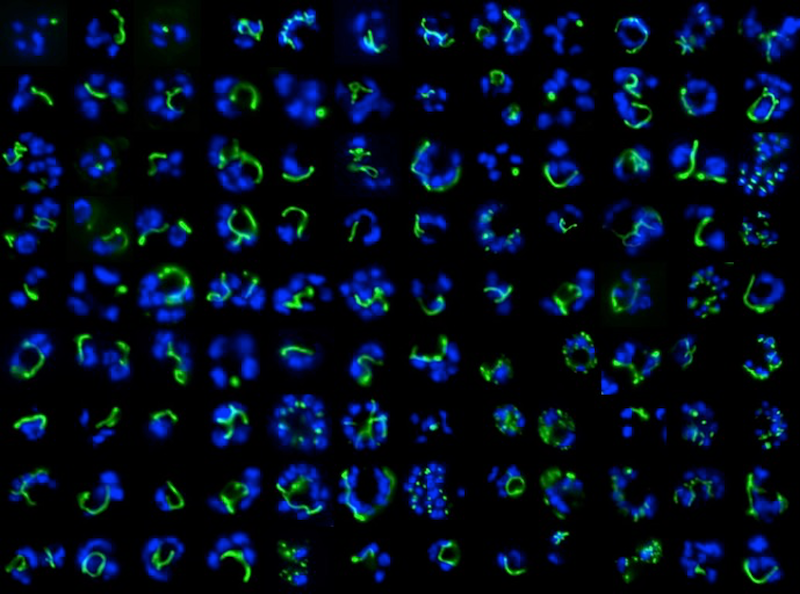 Epifluorescence microscopy of the Plasmodium apicoplast organelle during elongation
and branching (green = apicoplast, blue = dividing nuclei).
Epifluorescence microscopy of the Plasmodium apicoplast organelle during elongation
and branching (green = apicoplast, blue = dividing nuclei).
Dynamic motion of hemozoin crystals in the digestive vacuole of blood-stage Plasmodium parasites
References
Biosciences MB/BC students underlined
- García-Guerrero AE, Marvin RG, Blackwell AM, and Sigala PA. “Biogenesis of Cytochromes c and c1 in the Electron Transport Chain of Malaria Parasites.” ACS Infect. Dis. (2025) 11:4, 813-826, PMID: 39481007
- Blackwell AM, Jami-Alahmadi Y, Nasamu AS, Kudo S, Senoo A, Slam C, Tsumoto K, Wohlschlegel JA, Caaveiro JMM, Goldberg DE, and Sigala PA. “Malaria parasites require a divergent heme oxygenase for apicoplast gene expression and biogenesis.” eLife(2024) 13:RP100256, PMID: 39660822, PMCID: PMC11634067
- Loveridge KM and Sigala PA. “Identification of a divalent metal transporter required for cellular iron metabolism in malaria parasites.” Proc. Natl. Acad. Sci. (2024), 121: e2411631121, PMID: 39467134
- Espino-Sanchez TJ*, Wienkers H*, Marvin RG*, Nalder SA, García-Guerrero AE, VanNatta PE, Jami-Alahmadi Y, Blackwell AM, Whitby FG, Wohlschlegel JA, Kieber-Emmons MT, Hill CP, and Sigala PA. “Direct Tests of Cytochrome c and c1 Functions in the Electron Transport Chain of Malaria Parasites.” Proc. Natl. Acad. Sci. (2023) 120: e2301047120. PMID: 37126705, PMCID: PMC10175771.
- Okada M and Sigala PA. “The interdependence of isoprenoid synthesis and apicoplast biogenesis in malaria parasites.” PLoS Pathog. (2023) 19(10):e1011713. PMID: 37883328. PMCID: PMC10602226.
- Okada M, Rajaram K, Swift RP, Mixon A, Maschek JA, Prigge ST, and Sigala PA. “Critical Role for Isoprenoids in Apicoplast Biogenesis by Malaria Parasites.” eLife(2022) 11:e73208. PMID: 35257658, PMCID: PMC8959605.
- Feng H, Patel D, Magda JJ, Geher S, Sigala PA, and Gale BK. “Multiple-Streams Focusing-Based Cell Separations in High Viscoelasticity Flow.” ACS Omega(2022) 7:41759-41767. PMID: 36406492, PMCID: PMC9670260.
- Falekun S, Sepulveda J, Yasaman JA, Park H, Wohlschlegel JA, and Sigala PA. “Divergent Acyl Carrier Protein Decouples Mitochondrial Fe-S Cluster Biogenesis from Fatty Acid Synthesis in Malaria Parasites” eLife (2021)10:e71636. PMID: 34612205, PMCID: PMC8547962.
- Okada M, Guo P, Nalder SA, and Sigala PA. “Doxycycline has Distinct Apicoplast-Specific Mechanisms of Antimalarial Activity.” eLife (2020) 9: e60246. PMID: 33135634, PMCID: PMC7669263.
- Goldberg DE and Sigala PA (2017) Plasmodium heme biosynthesis: To be or not to be essential? PLoS Pathogens, 13(9): e1006511. PMID: 28957449
