Katharine S. Ullman
Professor of Oncological Sciences and Adjunct Professor of Biochemistry
Cell Division, Nuclear Transport, Nuclear assembly, DNA damage, Cytokinesis

Molecular Biology Program
Education
B.A. Northwestern University
Ph.D. Stanford University
Research
My lab is interested in the processes that guide reformation of nuclear architecture following mitosis and how this is integrated with genome surveillance and cell division regulation. Our long-term goals are to use this information to better understand how deregulation of these events contributes to tumor formation and progression and, ultimately, to identify targets that can be used to find new inhibitors of cancerous cell growth.
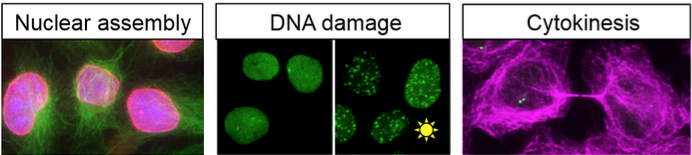
At interphase, the nucleus and cytoplasm of a eukaryotic cell provide unique environments for specialized processes. The nuclear envelope, formed by two membrane bilayers, provides a barrier between these two main compartments, while at the same time, nuclear pore complexes provide channels through which regulated traffic can take place across this barrier. We are interested in learning how individual components of the pore function both in transport and also in non-canonical roles, such as the response to DNA damage.
When a higher eukaryotic cell divides, the nucleus undergoes dramatic morphological remodeling and nuclear and cytoplasmic contents intermix. The nuclear pore complex also disperses at this time. As soon as chromosomes begin to separate in mitosis, the nuclear envelope begins to reform. We are studying how pore proteins, as well as other factors, participate in processes critically important to coordinating nuclear envelope formation and establishing normal nuclear architecture.
We are also capitalizing on the discovery that loss of post-mitotic function of the pore protein Nup153 results in activation of the abscission checkpoint, halting progression through cytokinesis, the last step of cell division. Working collaboratively with the Sundquist lab, we are studying how the role for ESCRT factors in cytokinetic abscission is regulated by this checkpoint.
References
-
von Appen A.*, LaJoie D.*, Johnson I.E.*, Trnka M.J., Pick S.M., Burlingame A.L., Ullman K.S.+, Frost A.+ (2020) LEM2 phase separation promotes ESCRT-mediated nuclear envelope reformation. Nature 582:115-118. (*contributed equally +corresponding authors)
-
Sadler, J., Wenzel, D., Williams, L., Guindo-Martinez, M., Alam, S., Mercader, J., Torrents, D., Ullman, K., Sundquist, W., and Martin-Serrano, J. (2018) A cancer-associated polymorphism in ESCRT-III disrupts the abscission checkpoint and promotes genome instability, PNAS, 115:E8900-E8908
-
Mackay, D.R., Howa, A.C., Werner, T.L., and Ullman, K.S. (2017) Nup153 and Nup50 promote recruitment of 53BP1 to DNA repair foci by antagonizing BRCA1-dependent events, J. of Cell Science, 130:3347
-
Gu, M.*, LaJoie, D*., Chen, O.S.*, von Appen, A., Ladinsky, M.S., Redd, M.J., Nikolova, L., Bjorkman, P.J., Sundquist, W. I.+, Ullman, K.S. +, and Frost, A.+ (2017) LEM2 recruits CHMP7 for ESCRT-mediated nuclear envelope closure in fission yeast and human cells, PNAS, 114:E2166. *contributed equally +corresponding authors
-
LaJoie, D. and Ullman, K.S. (2017) Coordinated events of nuclear assembly, Current Opinion in Cell Biology, 46:39.
-
Sundquist, W.I., and Ullman, K.S. (2015) CELL BIOLOGY. An ESCRT to seal the envelope, Science, 348:1314-5
-
Mackay, D.R., and Ullman, K.S. (2015) ATR and a Chk1-Aurora B pathway coordinate postmitotic genome surveillance with cytokinetic abscission, Molecular Biology of the Cell, 26:2217-26
-
Fay, M.M., Clegg, J.M., Uchida, K.A., Powers, M.A., and Ullman, K.S. (2014) Enhanced Arginine Methylation of Programmed Cell Death 4 during Nutrient Deprivation Promotes Tumor Cell Viability, Journal of Biological Chemistry, 289:17541-52
-
Chow, K.-H., Elgort, S.W., Dasso, M., Powers, M.A., and Ullman, K.S. (2014) The SUMO proteases SENP1 and SENP2 play a critical role in nucleoporin homeostasis and nuclear pore complex function, Molecular Biology of the Cell, 25:160-8
-
Makise, M., Mackay, D.R., Elgort, S., Shankaran, S.S., Adam, S.A., and Ullman, K.S. (2012) The Nup153-Nup50 interface and its role in nuclear import, Journal of Biological Chemistry, 287:38515-22
-
Chow, K.-H., Factor, R.E., and Ullman, K.S. (2012) The Nuclear Envelope Environment and Its Cancer Connections, Nature Reviews Cancer, 12:196-209
-
Powers, M.A.#, Fay, M.M.#, Factor, R.E., Welm, A.L*., and Ullman, K.S.* (2011) PRMT5 accelerates tumor growth by arginine methylation of the tumor suppressor PDCD4, Cancer Research, 71:5579-87 *equal contribution
-
Mackay, D., Makise, M., and Ullman, K.S. (2010) Defects in nuclear pore assembly lead to activation of an Aurora B-mediated abscission checkpoint, Journal of Cell Biology, 191:923-31
-
Mackay, D., Elgort, S., and Ullman, K.S. (2009) The nucleoporin Nup153 has separable roles in both early mitotic progression and the resolution of mitosis, Molecular Biology of the Cell, 20:1652-60
-
Higa, M., Alam, S., Sundquist, W., and Ullman, K.S. (2007) Molecular characterization of the Ran-binding zinc finger domain, Journal of Biological Chemistry, 282:17090-100
-
Prunuske, A., Liu, J., Elgort, S., Joseph, J., Dasso, M., and Ullman, K.S. (2006) Nuclear envelope breakdown is coordinated by both Nup358/RanBP2 and Nup153, two nucleoporins with zinc finger modules, Molecular Biology of the Cell, 17:760-760
-
Prunuske, A., and Ullman, K.S. (2006) The Nuclear Envelope: form and reformation, Current Opinion in Cell Biology, 18:108-16
